Now Reading: CDC’s vaccine advisory panel, with new members picked by RFK Jr., to vote on measles, hepatitis B shots
-
01
CDC’s vaccine advisory panel, with new members picked by RFK Jr., to vote on measles, hepatitis B shots
CDC’s vaccine advisory panel, with new members picked by RFK Jr., to vote on measles, hepatitis B shots
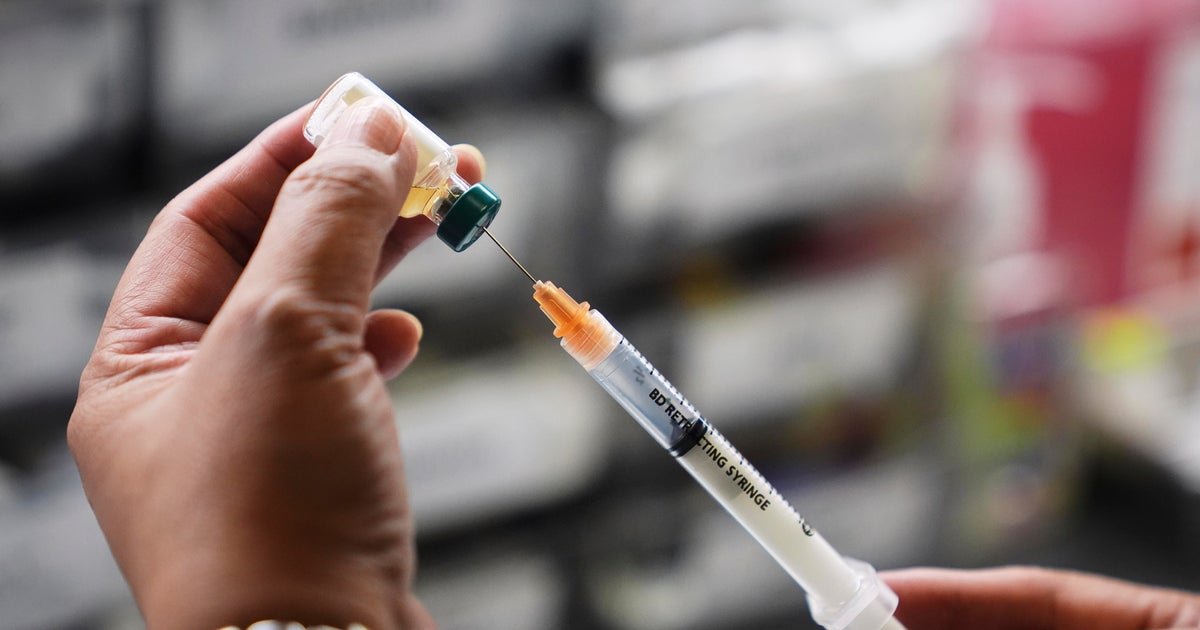
Committee shares proposed MMRV vaccine recommendation ahead of vote
The proposed recommendation language, which will later go to a vote, was presented as follows:
“The pediatric vaccine schedule should be updated to reflect the following change:
- For measles, mumps, rubella and varicella vaccines given before age 4 years, the combined MMRV vaccine is not recommended.
- Children in this age group should receive separate measles, mumps, and rubella vaccine and varicella vaccine (MMR+V).”
Currently, the CDC says the MMRV vaccine may be used in that age range if preferred by parents or caregivers to reduce the number of shots.
Measles vaccine presentations wrap with questions about febrile seizure risk
After presentations on the background and potential risks of febrile seizures with an MMRV vaccine — which combines the MMR and varicella shots — versus separate MMR and varicella shots, members discussed whether guidance should be changed.
Some members were satisfied with the current recommendations, which make clear the slightly higher risks of febrile seizures with the combined shot.
“I think the current wording is appropriate,” Dr. Cody Meissner, a pediatrics professor who previously served as a member of the Food and Drug Administration’s vaccines panel, said.
Others were not assured, saying there were too many assumptions being made. Some also pointed out that even if there are no worries about the physical impact of febrile seizures, the mental impact the event can have on children and families could affect vaccine compliance.
Dr. Richard Haupt, the head of global medical and scientific affairs, vaccines and infectious diseases at Merck, the vaccine’s manufacturer, noted the recent decline in vaccination rates among kindergarten children and highlighted the need to make guidance clear for the public.
“Considering these trends, any policy decision that compromises the clarity or consistency of vaccination guidance has the potential to further diminish public confidence,” he said.
ACIP chair says he was never contacted by ousted CDC director about vaccine concerns
During Thursday’s meeting, ACIP chair and biostatistician Martin Kulldorff noted the removal of CDC Director Dr. Susan Monarez and other members of CDC leadership, including former chief medical officer Dr. Debra Houry — both of whom testified a day prior about their removal.
“On vaccines, this committee is the key adviser to the CDC director, but during her short tenure, she never contacted me as the ACIP chair about any of her questions or concerns, which would have been natural if she had such concerns, neither was I contacted by any of the three CDC leaders, who subsequently resigned,” he said.
Kulldorff said the CDC leadership left citing divergent opinions about vaccines, but Monarez said she was also pressured by Kennedy to fire career experts without cause and approve vaccine recommendations without scientific evidence.
Kulldorff also said the American Academy of Pediatrics ended its participation with the committee and ignored invitations for open discussion about vaccines.
Last month, for the first time in 30 years, the AAP shared guidance that differed from the U.S. government. The organization said it is “strongly recommending” COVID-19 shots for children ages 6 months to 2 years old. Under Kennedy, the CDC doesn’t recommend COVID-19 shots for healthy children of any age. Instead, it says parents may get their kids vaccinated in consultation with physicians.
ACIP members highlight vaccine views during meeting roll call: “I’m not anti-vax”
As the meeting kicked off Thursday with a roll call, ACIP members presented some of their career backgrounds — and some took the time to highlight their views on vaccines.
Dr. Evelyn Griffin, an obstetrician and gynecologist based in Louisiana who was added to the panel earlier this week, said she would call herself “pro-informed consent.”
“Because of medical ethics, for discussing risk benefits and alternatives with the patient,” she said. “During the pandemic, I myself was COVID vaccinated.”
An earlier Kennedy pick, Dr. Joseph Hibbeln, who is retired from the National Institutes of Health, said he has a “neutral mind towards vaccines” and is “approaching this with a scientific equity.”
Dr. James Pagano, described by Kennedy as a “strong advocate for evidence-based medicine,” disclosed that he’s been vaccinated numerous times against various diseases.
“So I’m not anti-vax,” he said. “I am pro-intelligent and informed utilization of these potentially life-saving medications in a manner that reflects the current state of the art regarding their benefits, the target populations, optimal dosing and timing and, yes, of their potential adverse effects in some people.”
Science, politics and the future of vaccination in spotlight
The ACIP is convening under an unaccustomed spotlight. The committee usually attracts little attention as it deliberates vaccine schedules and eligibility, but suddenly finds itself navigating political scrutiny, public skepticism and internal upheaval.
The stakes extend well beyond the technical details of dosing intervals or eligibility cutoffs. The panel’s decisions could reshape public trust in childhood vaccines, restrict access to vaccines through Medicaid and Medicare, and signal whether scientific consensus or political pressure will steer the nation’s vaccination strategy.
Read more here.
Current recommendations for hepatitis B and MMRV vaccines
For hepatitis B, the CDC currently recommends the first dose within 24 hours after birth. Universal infant vaccination became the norm in 1991 after data showed too many cases of hepatitis B among pregnant women were missed during prenatal care.
A universal birth dose acts as a safety net, protecting infants whose parent’s infection might have been missed. Before birth-dose policies, the U.S. saw an estimated 1,000 preventable infections in newborns each year.
To protect against measles, mumps, rubella and varicella (or chickenpox), there are two options: a combo MMRV vaccine or separate MMR and varicella vaccines.
The CDC currently recommends a two-dose series beginning at age 12 to 15 months. However, the combination vaccine contains certain risks for younger age groups; specifically, the combo shot carries a slightly higher risk of fever-related “febrile” seizures when used as the first dose in young toddlers aged 12-23 months.
“For dose 1 in children age 12–47 months, it is recommended to administer MMR and varicella vaccines separately,” the CDC says, but adds MMRV may be used if preferred.
Former CDC director said she is “very nervous” about the upcoming childhood vaccine panel recommendations
While testifying at a Senate hearing Wednesday about why she was ousted as CDC director, Dr. Susan Monarez said she’s “very nervous” about the newly appointed ACIP members and what their recommendations might be.
“I know that the medical community has raised concerns about whether or not, again, they have the commensurate backgrounds to be able to understand the data, the evidence, and to evaluate it appropriately, but I certainly will be watching,” she said.
In another part of the hearing, Monarez said she refused to rubber-stamp vaccine recommendations without seeing the evidence behind them because she “built a career on scientific integrity.”
“My worst fear was that I would then be in a position of approving something that would reduce access to lifesaving vaccines to children and others who need them,” she said.
Monarez added she is not aware of any scientific evidence to support changing the childhood vaccination schedule for measles, chicken pox and hepatitis B.
Kennedy’s newly appointed ACIP members include allies and vaccine critics
After firing all 17 of the committee’s previous members in June, Kennedy named eight new advisers to the ACIP, one of whom later withdrew. They include several allies he has worked with closely over the years and some have a history as vaccine critics.
Kennedy appointed the new members directly, breaking with the past practice of agency officials vetting potential experts before sending them to the secretary for approval.
Just this week, the group gained five new members, the HHS announced. The latest additions include some who have questioned established medical research on immunizations and the COVID-19 pandemic.
Panel to consider COVID vaccine recommendations Friday as West Coast states move ahead
A day before the ACIP meetings kicked off, a group of four West Coast states recommended that all adults and children in those states who want them can receive the COVID-19 vaccine and other common shots.
The announcement was made in a joint statement from Oregon Gov. Tina Kotek, Washington Gov. Bob Ferguson, California Gov. Gavin Newsom and Hawaii Gov. Josh Green, all Democrats, saying they were putting safety before politics.
The guidance, which aligns with mainstream medical groups like the American Academy of Family Physicians and American Academy of Pediatrics, comes amid confusion over the CDC’s messaging on vaccinations.