Now Reading: RFK Jr.’s new ACIP votes to recommend RSV shot for infants
-
01
RFK Jr.’s new ACIP votes to recommend RSV shot for infants
RFK Jr.’s new ACIP votes to recommend RSV shot for infants


RFK Jr. to defund global vaccine group, citing safety concerns
Health Secretary Robert F. Kennedy Jr. said funding is halted for an organization that offers free vaccines for meningitis, malaria, and other diseases.
unbranded – Newsworthy
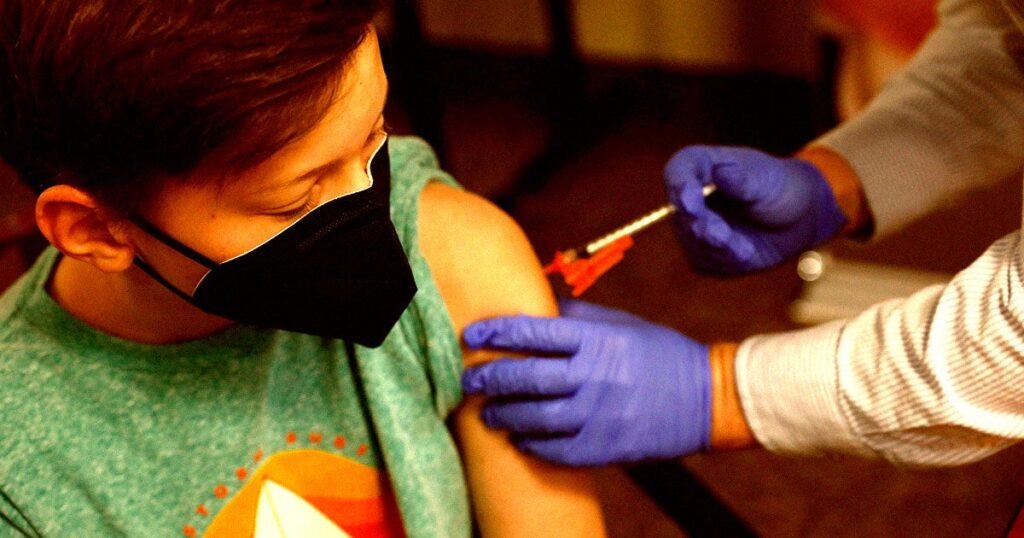
In their first vote since appointed by Health and Human Services Secretary Robert F. Kennedy Jr., the eight members of a vaccine committee voted to recommend a shot that protects infants against respiratory syncytial virus, or RSV.
Clesrovimab, a monoclonal antibody created by pharmaceutical giant Merck, is recommended for use in infants younger than 8 months born during or entering their first RSV season.
Martin Kulldorff, who led the meeting June 26, recently served as an expert witness for plaintiffs who accused Merck of concealing the risks of Gardasil, a vaccine used to prevent cancer from human papillomavirus, or HPV.
The eight new members of the Advisory Committee on Immunization Practices met for the first time June 25. Kennedy fired all 17 original members of the committee on June 9 and appointed its new members a few days later. Given the inclusion of vaccine skeptics, the approval comes as a surprise to close watchers of the panel.
Experts from the Centers for Disease Control and Prevention’s workgroup that presented data on the drug universally supported the drug. Two panel members still voted against it, however.
RSV infects nearly everyone by the time they’re 2. It causes cold symptoms, affecting the breathing passages and lungs, according to the CDC. In the United States, about 58,000 children younger than 5 are hospitalized for RSV each year, and several hundred die.
Merck’s shot is the second RSV monoclonal antibody of its kind on the market. The first, a shot from Sanofi and AstraZeneca called Beyfortus, was approved by the Food and Drug Administration in July 2023 and prevents RSV lower respiratory tract disease in infants entering or during their first RSV season.
It was the first of its kind to be widely available to everyone beyond a small population of immunocompromised children. Data presented at the June 25 ACIP meeting found the shot reduced hospitalizations by about 47% in newborns up to 2 months old, the population at highest risk of hospitalization.
Dr. Cody Meissner, who voted to recommend the Merck shot, said June 25 that the result data from Beyfortus in the past RSV season was an “astonishing accomplishment.”
The Beyfortus shot was recommended by ACIP in 2023 for babies under 9 months old who are entering their first RSV season. It’s also recommended for babies 8 to 19 months who are at increased risk of severe disease if they’re entering their second season of RSV.
Parents will have the choice between the two shots. If they receive Beyfortus, they’re not recommended to get the new monoclonal antibody by Merck.
Pregnant patients also have access to Pfizer’s RSV vaccine Abrysvo, which is recommended for pregnant people to protect newborns from lower respiratory infections. The shot is approved for use during the 32nd and 26th weeks of pregnancy during the RSV season, which typically starts in September and runs through January in the United States.
Adrianna can be reached at adrodriguez@usatoday.com.