Now Reading: What are the latest COVID vaccine guidelines for this summer?
-
01
What are the latest COVID vaccine guidelines for this summer?
What are the latest COVID vaccine guidelines for this summer?

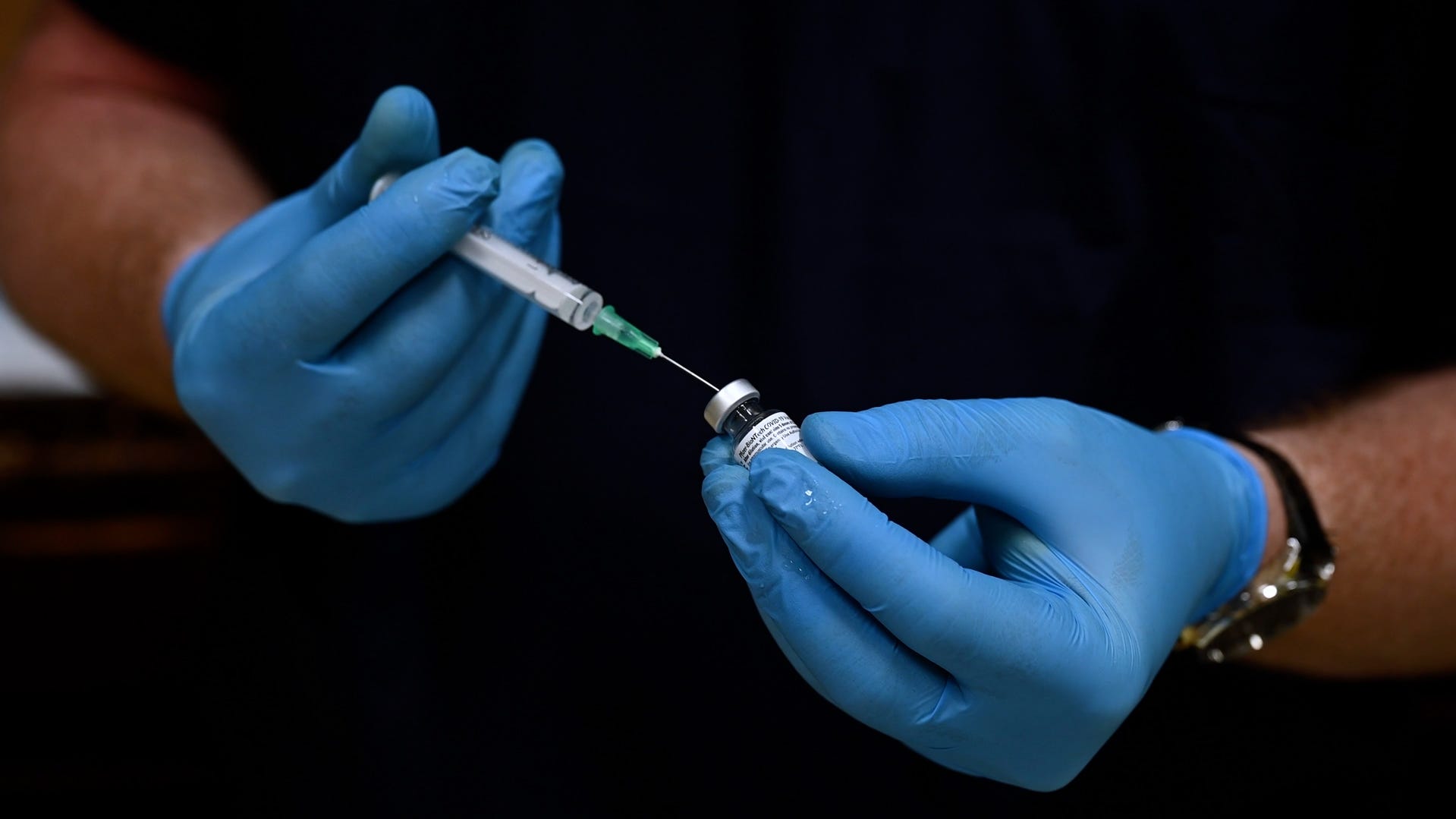
RFK Jr. says COVID-19 vaccine no longer recommended for some
The COVID-19 vaccine is no longer recommended for healthy children and pregnant women, HHS Secretary Robert F. Kennedy Jr. says.
- The NB.1.8.1 COVID-19 variant is now the dominant strain in the U.S., exhibiting symptoms similar to previous variants, including a uniquely severe sore throat.
- The FDA updated warning labels for mRNA COVID-19 vaccines to include information about the risk of myocarditis.
- Despite the U.S. policy changes, WHO and other organizations continue to recommend COVID-19 vaccination for vulnerable populations.
As a new COVID-19 variant takes over in the U.S., guidance surrounding vaccines has become increasingly confusing.
Changes in vaccination guidelines, ever-evolving variants and strains, along with threats to health insurance, have sent average Americans looking for the latest recommendations as members of the federal government often conflict with independent medical agencies and healthcare professionals.
In the two weeks leading up to June 21, the Centers for Disease Control and Prevention (CDC) reported just shy of 14,500 positive COVID tests, and while hospitalizations and deaths are fortunately down significantly since the pandemic’s peak, vulnerable people are still grappling with limiting their risk amid changing practices.
Having trouble keeping track of variants and vaccines? Here’s what we know.
What is the new NB.1.8.1 COVID variant?
NB.1.8.1 is one of the latest variants of COVID-19, a “slightly upgraded version” of the LP.8.1 variant that is prominent right now, Subhash Verma, microbiology and immunology professor at the University of Nevada, Reno, previously told USA TODAY in May.
Verma previously stated that NB.1.8.1 may be transferred more easily than LP.8.1. Additionally, he noted that NB.1.8.1 can evade antibodies created by vaccines or past infections more easily than LP.8.1.
In early April, NB.1.8.1 accounted for 0% of COVID cases in the U.S. In the two weeks ending June 21, it accounted for the majority of cases at 43%, according to the CDC.
The variant has similar symptoms to other strains, including fever or chills, cough, shortness of breath or difficulty breathing, sore throat, congestion or a runny nose, new loss of taste or smell, fatigue, muscle or body aches, headache, nausea or vomiting. One of its more unique features is “razor blade throat,” reported by patients as an exceptionally sore throat.
RFK and HHS change COVID vaccine guidance
Health and Human Services (HHS) Secretary Robert F. Kennedy Jr. said on May 27 that the COVID-19 vaccine would no longer be included in the CDC’s recommended immunization schedule for healthy children and pregnant women, a move that broke with previous expert guidance and bypassed the normal scientific review process.
Under the changes, the only people who will be recommended for COVID-19 vaccines are those over 65 and people with existing health problems. This could make it harder for others who want the COVID-19 vaccine to get it, including health care workers and healthy people under 65 with a vulnerable family member or those who want to reduce their short-term risk of infection.
The American College of Obstetricians and Gynecologists (ACOG) and American Academy of Pediatrics (AAP), among other organizations, issued statements condemning the change, with the ACOG saying it was “…concerned about and extremely disappointed by the announcement that HHS will no longer recommend COVID-19 vaccination during pregnancy.”
“It is very clear that COVID-19 infection during pregnancy can be catastrophic and lead to major disability, and it can cause devastating consequences for families. The COVID-19 vaccine is safe during pregnancy, and vaccination can protect our patients and their infants after birth,” President Steven J. Fleischman said in a statement.
Insurance coverage typically follows federal recommendations, so anyone who is healthy and under 65 is likely to have to pay out of pocket to get the shot, which runs about $200, if they can get it. It’s not clear what insurance companies will do about the new recommendations.
AMA, AAP other organizations break from RFK and HHS on vaccines
The American Medical Association (AMA) and American Academy of Pediatrics (AAP), in partnership with other professional medical organizations, broke from RFK and HHS after this announcement, sharing plans to develop their own guidelines independent of the government organization.
In an open letter signed by 80 medical organizations across the country and published on June 25, the AMA called for physicians, healthcare networks and insurance companies to continue supporting “evidence-based immunizations to help prevent severe disease and protect public health.”
“Vaccines for influenza, RSV, and COVID-19 remain among the best tools to protect the public against these illnesses and their potentially serious complications—and physicians are among the most trusted voices to recommend them. We come together as physicians from every corner of medicine to reaffirm our commitment to these lifesaving vaccines,” the letter said.
“Recent changes to federal immunization review processes raised concerns across the medical and public health community. In this moment of uncertainty, physicians must align around clear, evidence-based guidance for patients.”
The AAP likewise said in a June 26 statement that it will “continue to publish its own evidence-based recommendations and schedules.”
AAP President Susan J. Kressly said the creation of federal immunization policy is “no longer a credible process,” adding, “…we’re not stepping back, we’re stepping up. The AAP will continue to publish our own immunization schedule just as we always have, developed by experts, guided by science, trusted by pediatricians and families across the country.”
These latest independent guidelines have yet to be released.
Vaccine committee adjourns without fresh recommendations
Meanwhile, the new Advisory Committee on Immunization Practices (ACIP) gathered for the first time on June 25 in a meeting that drew criticism from some experts.
RFK fired all 17 original members of the committee on June 9, replacing them with members that critics have called unqualified. Some of the members, like Kennedy, have a history of anti-vaccine advocacy, prompting backlash that had doctors and organizations calling for a delay in the meeting.
Anti-vaccine sentiments were repeated by ACIP Chair Martin Kulldorf at the meeting, who said the panel will be “investigating” MMR and childhood vaccines.
The CDC panel also reviewed data about COVID-19 vaccines, questioning their safety and effectiveness. They also raised questions about the study design, methodologies and surveillance monitoring systems behind the data, which Dr. Pamela Rockwell, clinical professor of family medicine at the University of Michigan Medical School, addressed as a standard of medical research.
“Our efforts, through a very robust system of checks and balances, are to create vaccines and vaccination programs that result in the most benefit with the least harm,” said Dr. Gretchen LaSalle, a family physician in Spokane, Washington, who represented the American Academy of Family Physicians.
Despite this, the committee didn’t vote on COVID-19 vaccine recommendations for the fall and isn’t expected to reconvene until “September/October,” according to the CDC website.
FDA updates warning label for COVID vaccines
The FDA likewise announced updated requirements for mRNA COVID-19 vaccine warning labels on June 25, which apply to Comirnaty by Pfizer Inc. and Spikevax by ModernaTX Inc. Prescribing information will now include warnings of the connection between the vaccines and a rare side effect that causes inflammation of the heart muscle and lining.
The new warning label discloses the risk of myocarditis, which appeared in 8 cases per 1 million people who got the 2023-2024 COVID shots between the ages of 6 months and 64 years old, mostly commonly among males aged 12 to 24. The previous label, which also disclosed the risk, said the problem mostly occurred in minors aged 12-17.
So, how do you protect yourself from NB.1.8.1 and other variants?
Despite the back-and-forth in the U.S., the World Health Organization (WHO) has kept its recommendation consistent. Currently approved COVID-19 vaccines are expected to remain effective against the NB.1.8.1 variant, it said.
In a webpage dated Jan. 7, the CDC advised that everyone over the age of six months get the 2024-2025 COVID-19 vaccine, specifically the 2024-2025 Moderna COVID-19 Vaccine. The page has since been updated with a banner, reading “COVID-19 vaccine recommendations have recently been updated for some populations. This page will be updated to align with the updated immunization schedule.” The original recommendations align with the WHO’s current guidelines.
WHO, AMA, AAP and existing standards recommend that people who have never received a COVID-19 vaccine, are age 65 and older, are immunocompromised, live at a long-term care facility, are pregnant, breastfeeding, trying to get pregnant, and/or want to avoid getting long COVID, should get the vaccine, especially.
Contributing: Greta Cross, Adrianna Rodriguez, USA TODAY